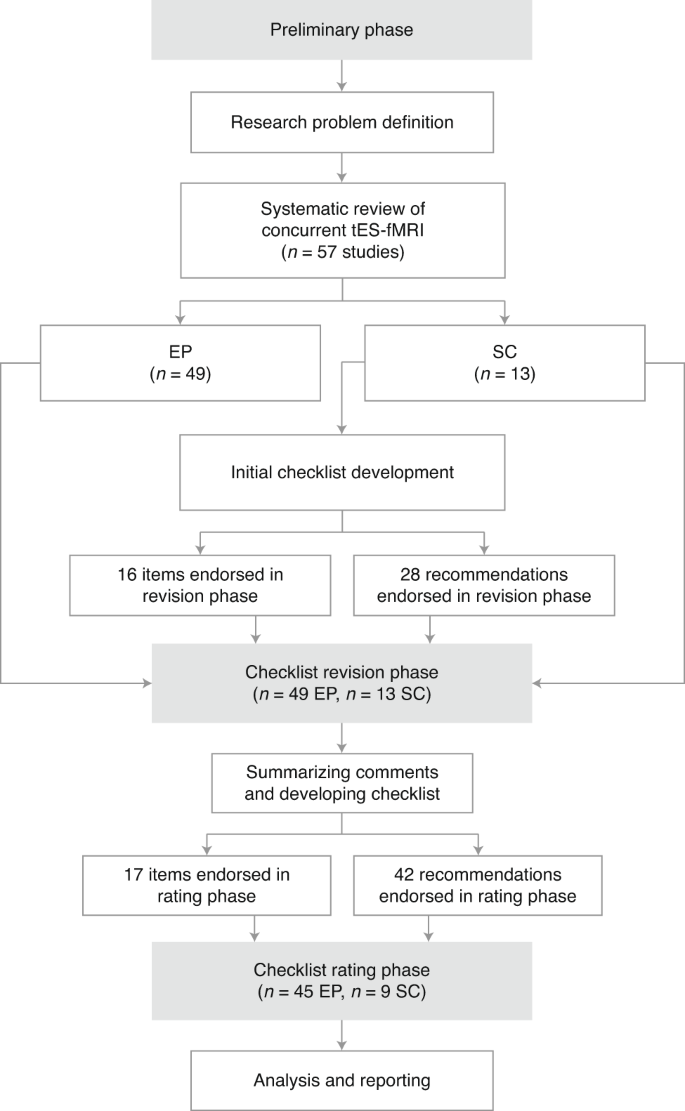
A checklist for assessing the methodological quality of concurrent tES-fMRI studies (ContES checklist): a consensus study and statement | Nature Protocols

Metabolism by Aldehyde Oxidase: Drug Design and Complementary Approaches to Challenges in Drug Discovery | Journal of Medicinal Chemistry

Task difficulty assessment : a contribution towards improved buildability through simplification. | Semantic Scholar