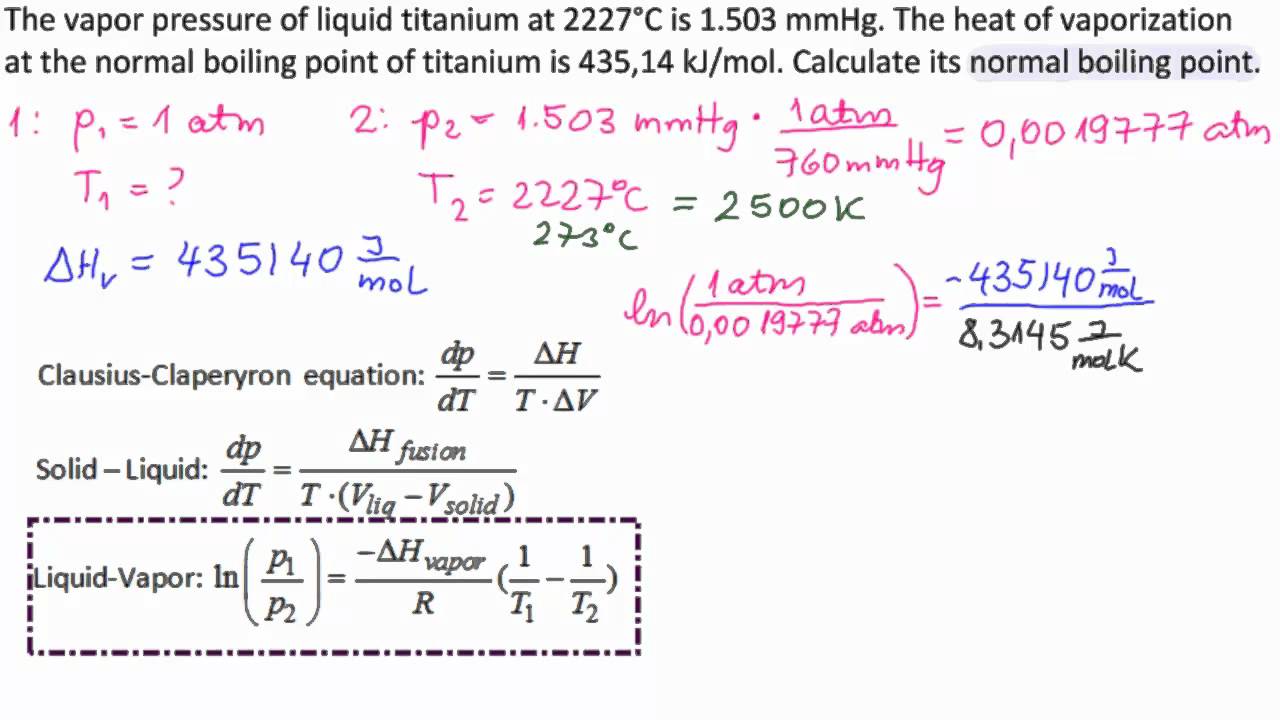
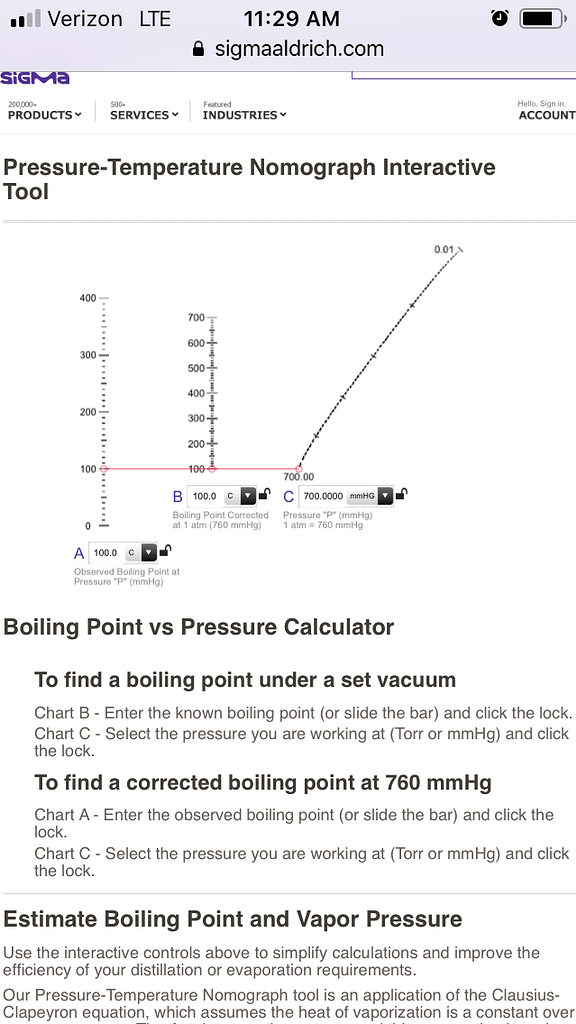
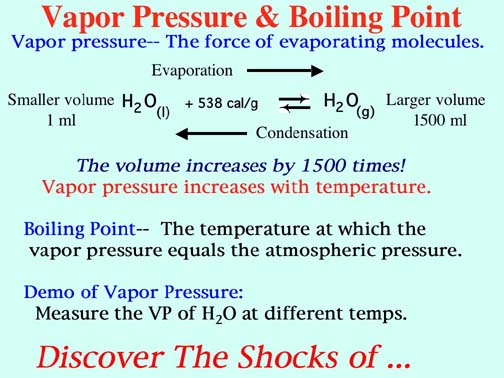
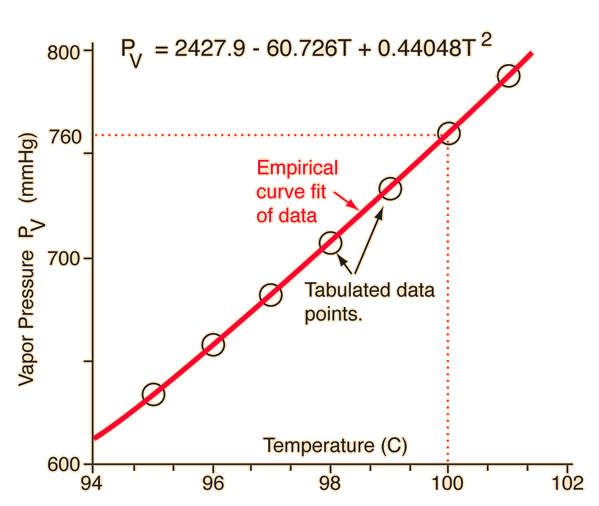
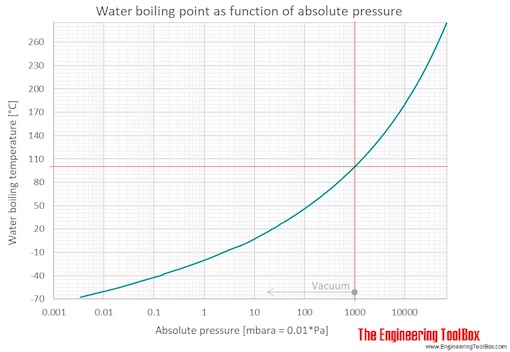
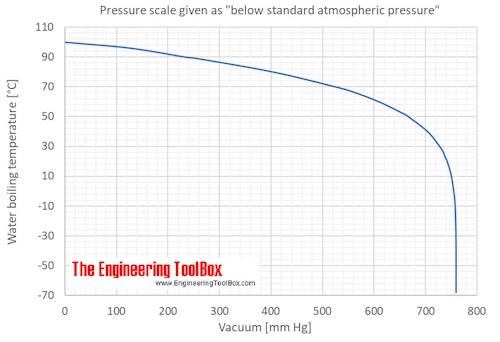
SOLVED: methanol has a normal boiling point of 64.6°C and a heat of vaporization H of 35.2 kJ/mol. What is the vapor pressure (in mm Hg) of methanol at 12.0°C

Methanethiol has a vapor pressure of 429 torr at −25 ∘c and a normal boiling point of 6.0 ∘c. find - Brainly.com
Problem Set #10 Assigned November 8, 2013 – Due Friday, November 15, 2013 Please show all work for credit To Hand in 1.