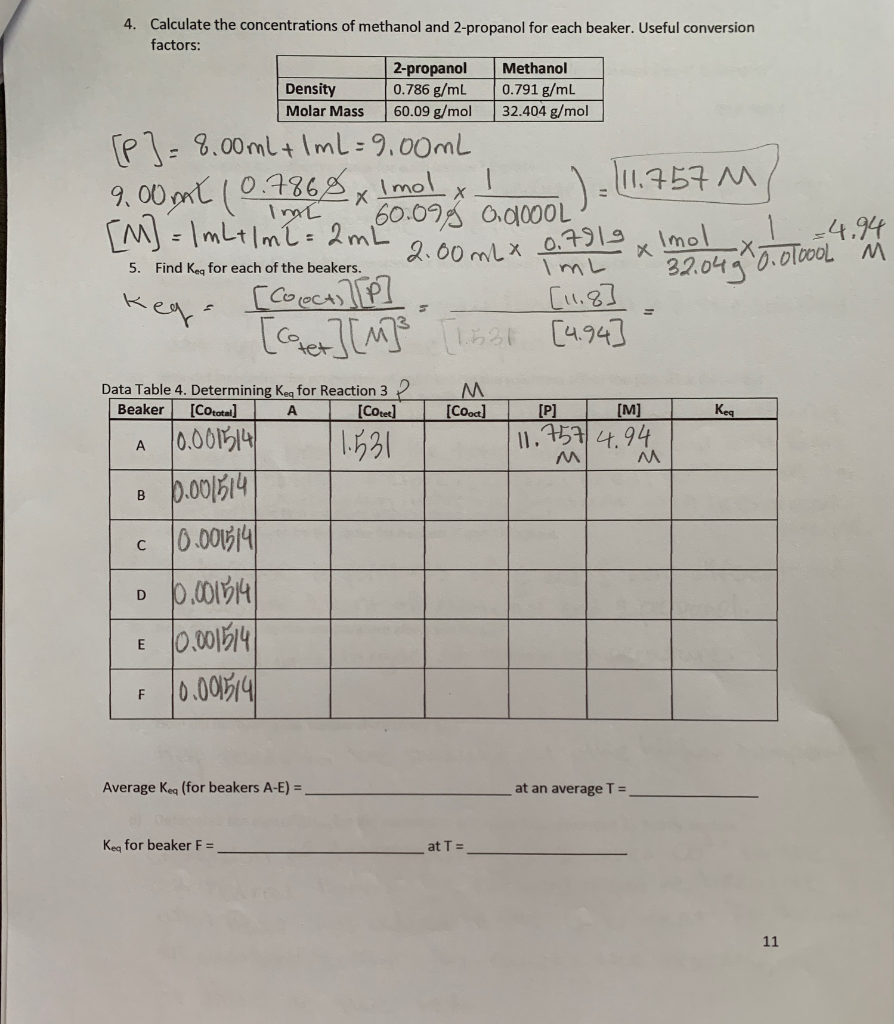
Methanol content (in grams per liter of 100% vol. alcohol, % and g per... | Download Scientific Diagram

At 293.2K, the density of a 60% aqueous solution of methanol is 0.8946 g/mL. Calculate volume of 1 mole of the solution.
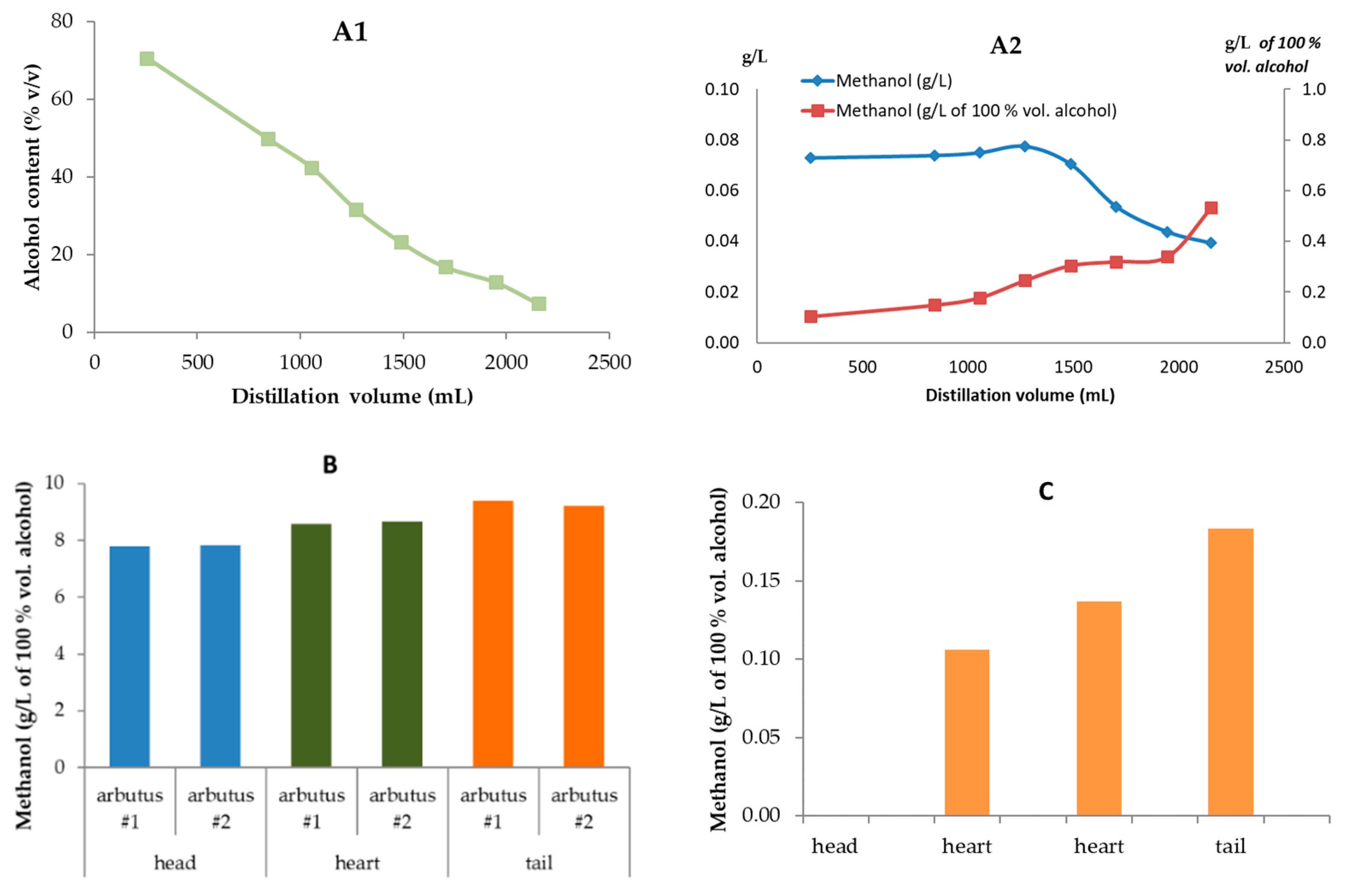
Processes | Free Full-Text | Methanol in Grape Derived, Fruit and Honey Spirits: A Critical Review on Source, Quality Control, and Legal Limits

Results of TPC calculation of antibacterial activity of gonad methanol... | Download Scientific Diagram

SOLVED: A solution is prepared by dissolving 20.2 mL of methanol (CH3OH) in 100.0 mL of water at 25 C The final volume of the solution is 118 mL. The densities of

SOLVED: Calculate the volume of 65.0 g of liquid methanol (wood alcohol) if its density is 0.791 g/mL.

If the density of methanol is 0.793 kg L^-1 , what is its volume needed for making 2.5 L of its 0.25 M solution?

Exercise1.pdf - Methanol has a density of 2.79 g/mL. If 16.5 mL of this methanol is added to water to make a 2000.0 mL solution, calculate the | Course Hero
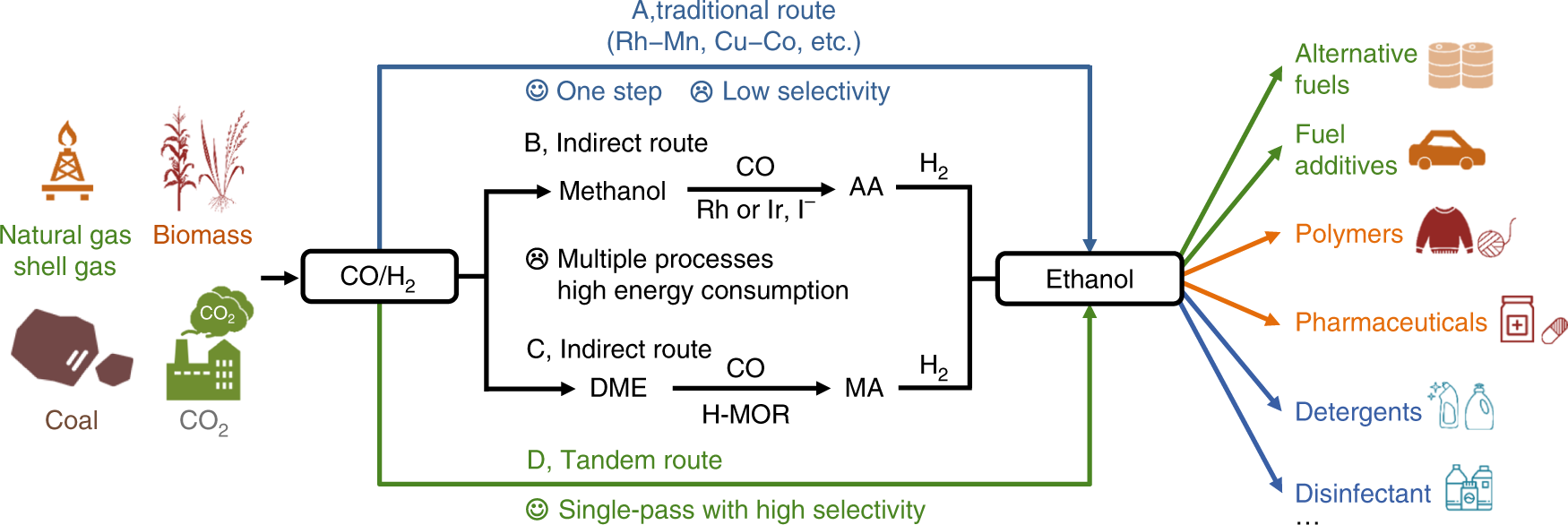
Single-pass transformation of syngas into ethanol with high selectivity by triple tandem catalysis | Nature Communications

SOLVED:The density of methanol, a colorless organic liquid used as solvent, is 0.7918 g / mL. Calculate the mass of 89.9 mL of the liquid.
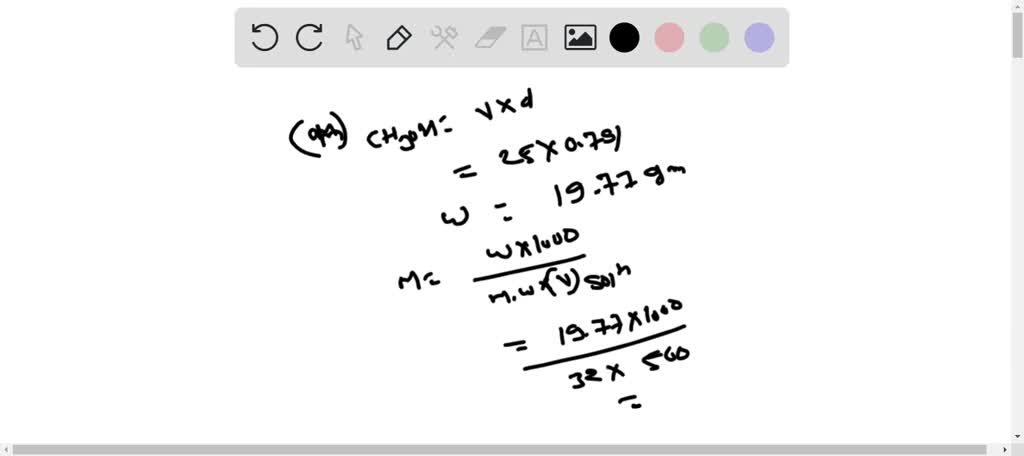
SOLVED: B. Preparation of a 25 % V/V aqueous methanol solution 1. Calculate the volume of methanol, in mL, needed to prepare 20.0 mL of a 25 % V/V solution in water.
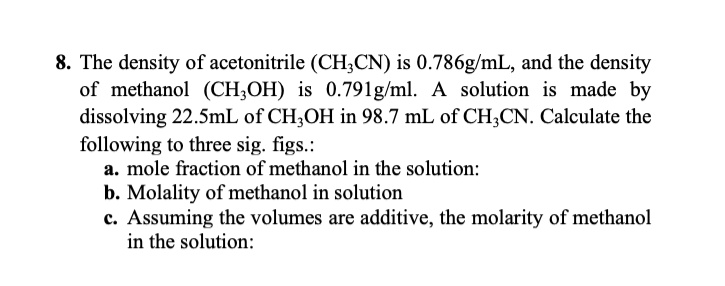
SOLVED: 8. The density of acetonitrile (CH;CN) is 0.786g/mL, and the density of methanol (CH;OH) 0.791g/ml: solution made by dissolving 22.SmL of CH;OH in 98.7 mL of CH;CN. Calculate the following to



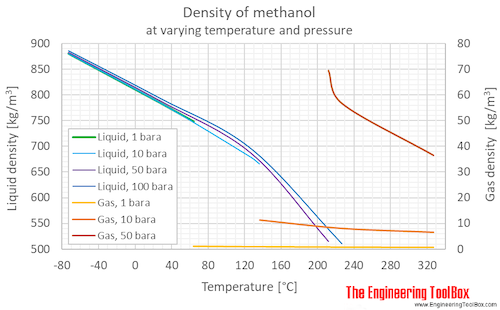
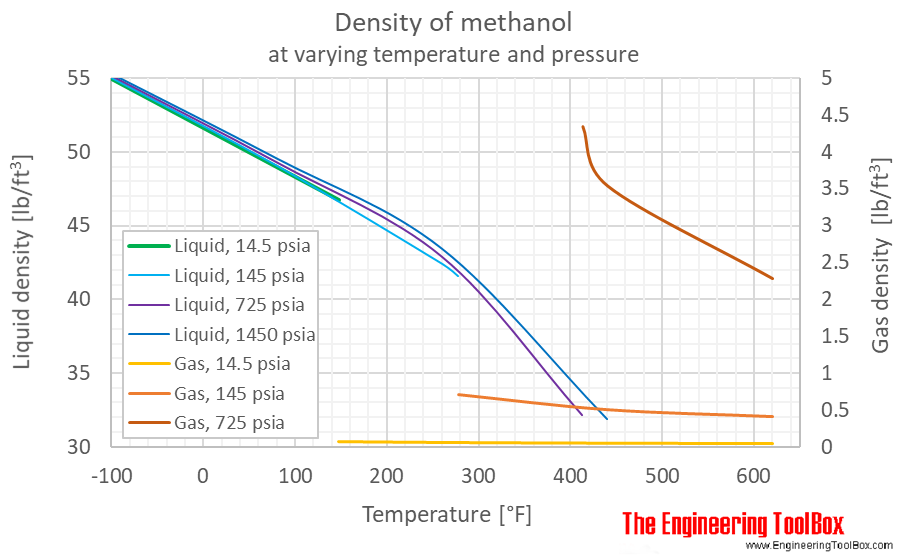
![ANSWERED] Calculate the osmotic pressure of each of... - Physical Chemistry ANSWERED] Calculate the osmotic pressure of each of... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/66524620-1657314910.1337452.jpeg)