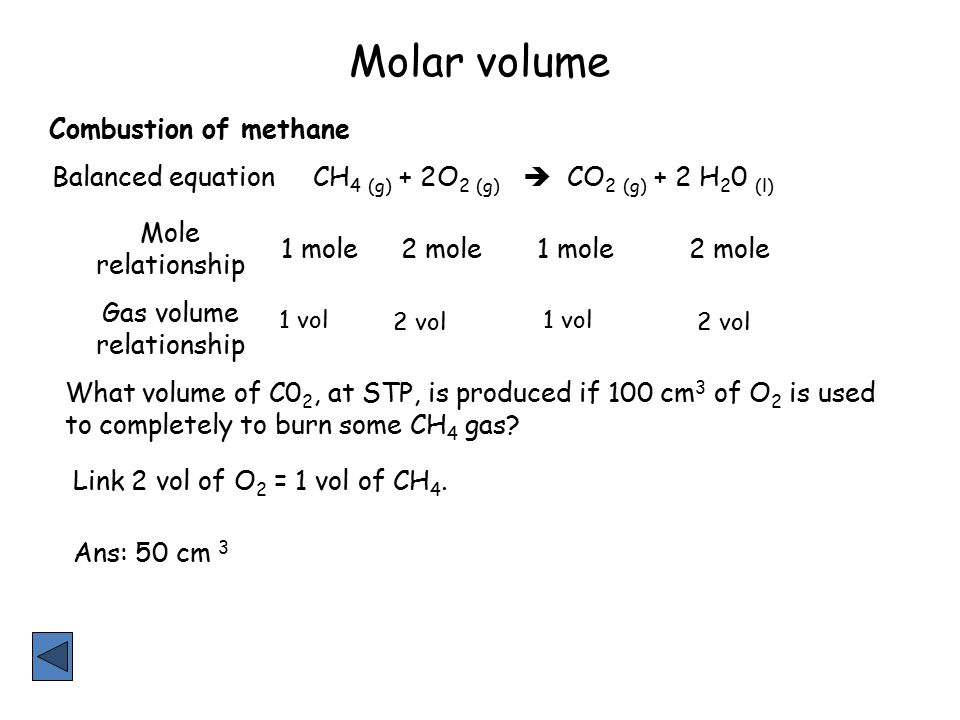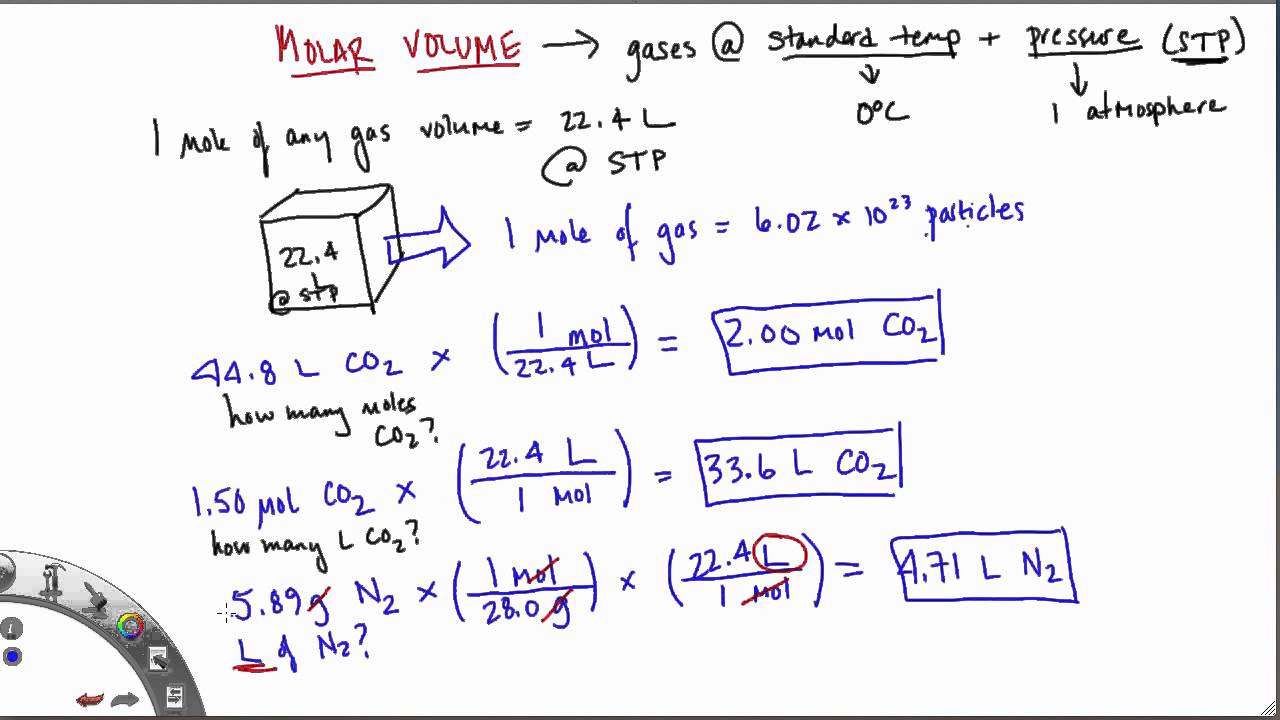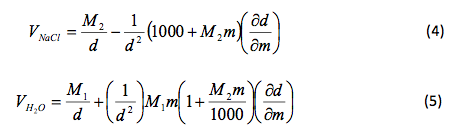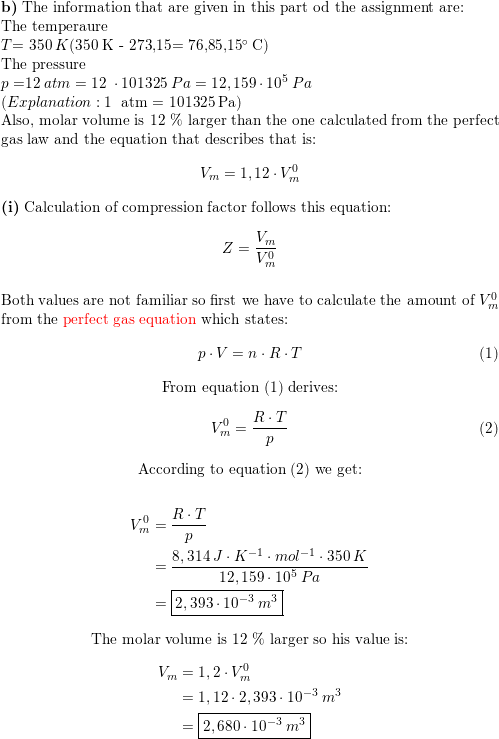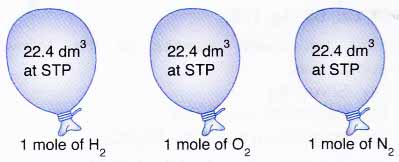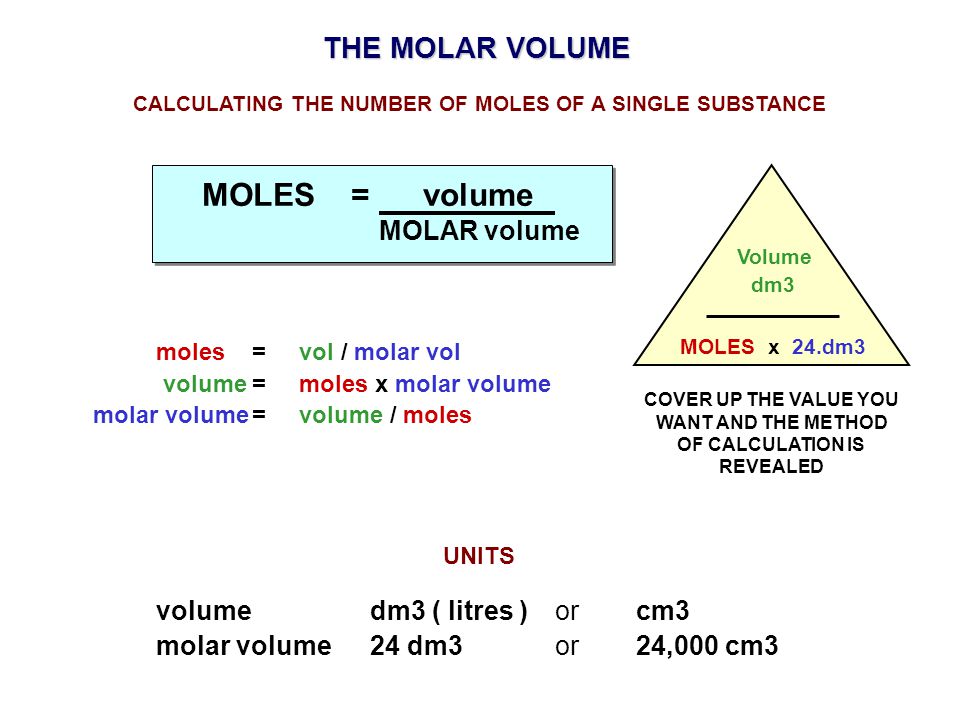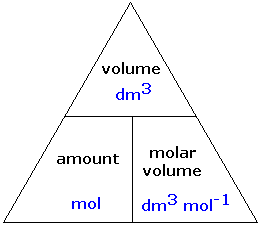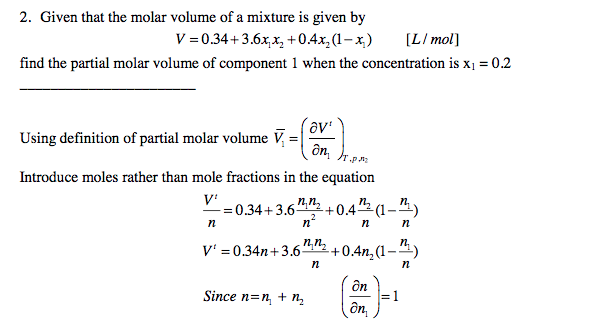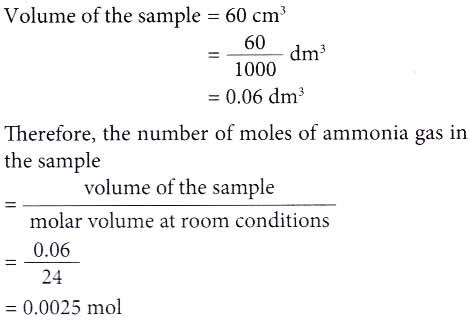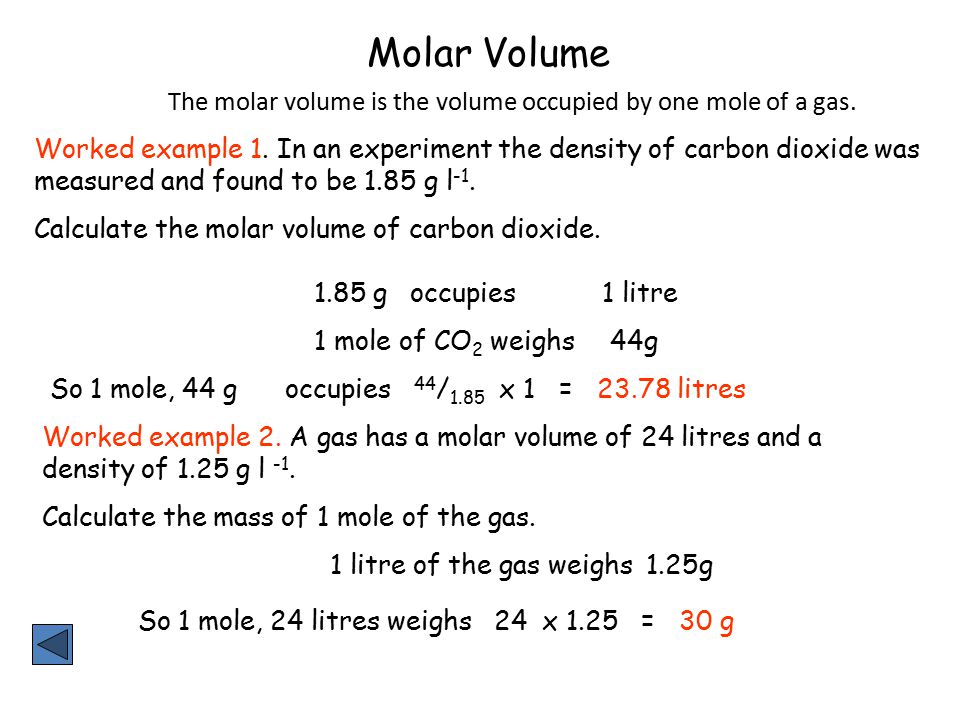
Mole and gas volume The molar volume of a gas is its volume per mole, litre mol-1. It is the same for all gases at the same temperature and pressure. The. -

molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises