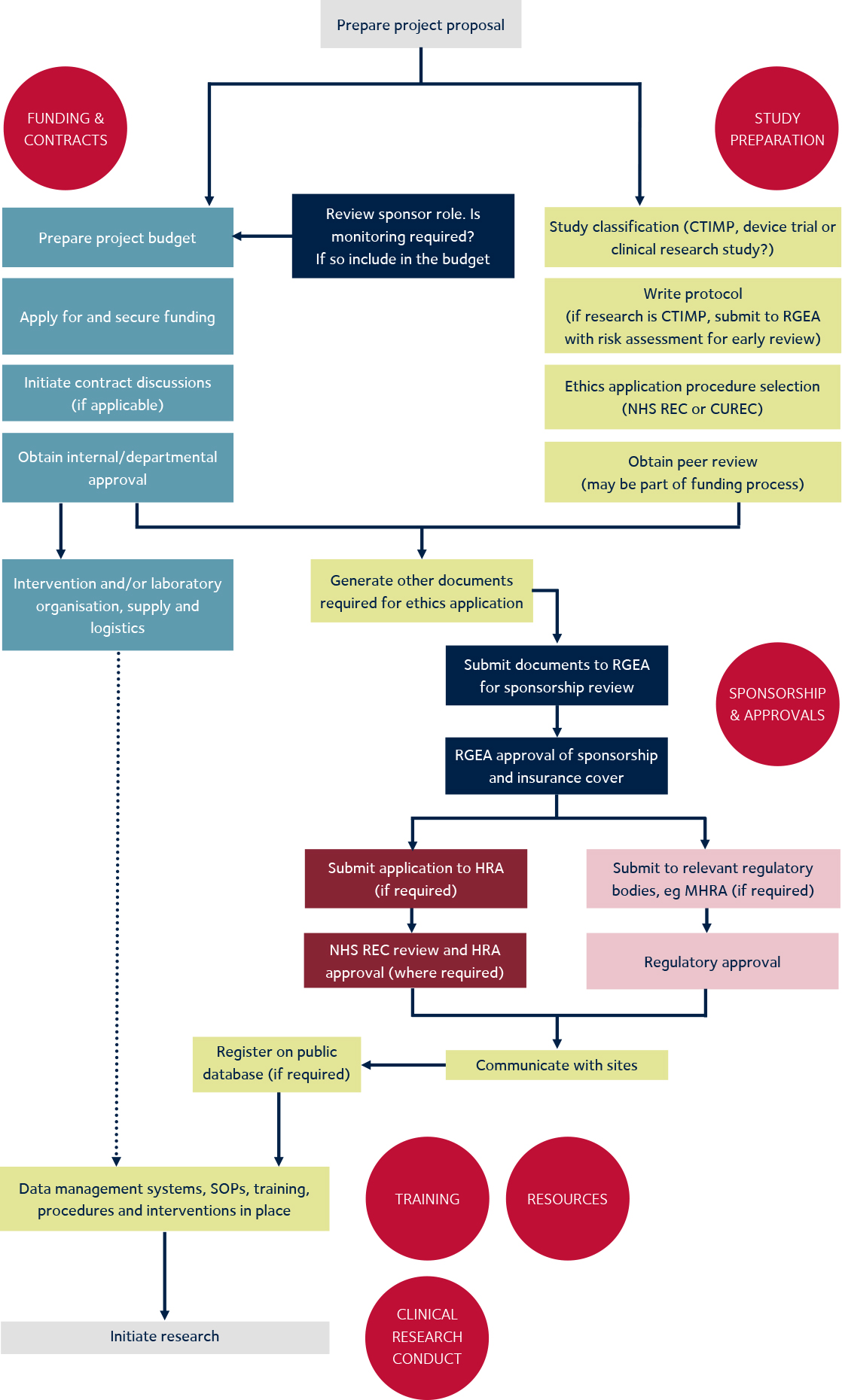
Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future – NLM Musings from the Mezzanine

NIA-Funded Active Alzheimer's and Related Dementias Clinical Trials and Studies | National Institute on Aging
Ten simple rules for maximizing the recommendations of the NIH data management and sharing plan | PLOS Computational Biology