
pH, pOH, H3O+ and OH- Introduction Calculations: Acids and Bases Chemistry Practice Problems. - YouTube
![Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. Calculate Ka/Kb, given the pH or pOH and the concentration. - ppt download Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. Calculate Ka/Kb, given the pH or pOH and the concentration. - ppt download](https://images.slideplayer.com/34/8310807/slides/slide_5.jpg)
Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. Calculate Ka/Kb, given the pH or pOH and the concentration. - ppt download
![SOLVED: 6. What is the correct expression for the calculation of pH? pH = log[1.0 x 10-14 b:pH = log([HTJ[OHT ]) pH = log[OH ] d: pH = log[OH ] pH = SOLVED: 6. What is the correct expression for the calculation of pH? pH = log[1.0 x 10-14 b:pH = log([HTJ[OHT ]) pH = log[OH ] d: pH = log[OH ] pH =](https://cdn.numerade.com/ask_images/f12f4e75d7de414ab6b3a75ee0a46607.jpg)
SOLVED: 6. What is the correct expression for the calculation of pH? pH = log[1.0 x 10-14 b:pH = log([HTJ[OHT ]) pH = log[OH ] d: pH = log[OH ] pH =
![SOLVED: pH= log [H3O*] pOH= log[OH ], pH POH = 14 = pKw, [HTJ[OH ]=10-14 = Kw (at 25*C) , [salt or CB] pKa = logKa, pKb logKb, pH pKa + log [ SOLVED: pH= log [H3O*] pOH= log[OH ], pH POH = 14 = pKw, [HTJ[OH ]=10-14 = Kw (at 25*C) , [salt or CB] pKa = logKa, pKb logKb, pH pKa + log [](https://cdn.numerade.com/ask_images/8005f0bfdafa41f0911fb8ef851f72e1.jpg)
SOLVED: pH= log [H3O*] pOH= log[OH ], pH POH = 14 = pKw, [HTJ[OH ]=10-14 = Kw (at 25*C) , [salt or CB] pKa = logKa, pKb logKb, pH pKa + log [



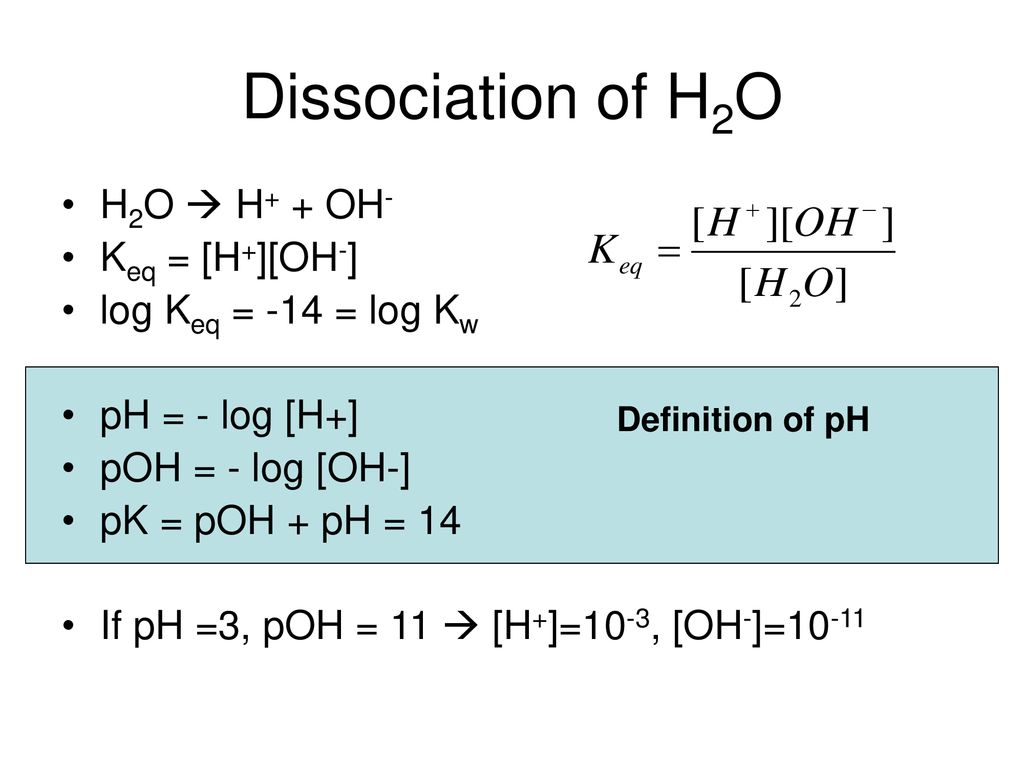
![Given [H+] or [OH-], Calculate pH & pOH - YouTube Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)
![Calculations of pH, pOH, [H+] and [OH-] Calculations of pH, pOH, [H+] and [OH-]](https://www.sciencegeek.net/Chemistry/taters/graphics/pHSchematic.gif)
![Solved If you know the [OH^-], how can you determine the pH | Chegg.com Solved If you know the [OH^-], how can you determine the pH | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2Fa28%2Fa28fcc20-bb68-4b04-bdc7-7a448f305470%2FphpfAvy6v.png)
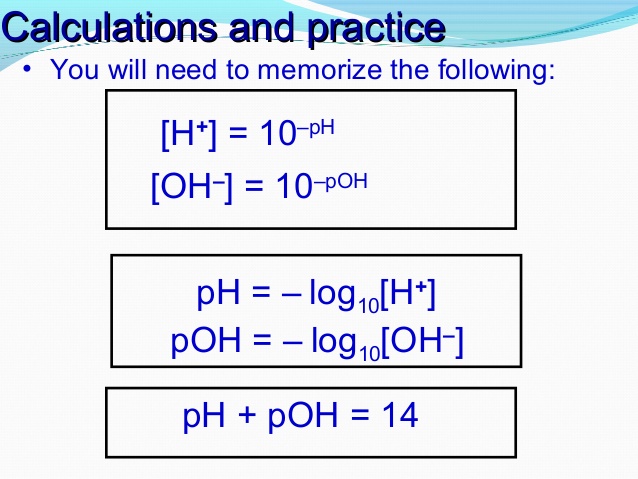


![pH Calculations pH = -log[H+] 10-pH = [H+] pOH = -log[OH-] - ppt download pH Calculations pH = -log[H+] 10-pH = [H+] pOH = -log[OH-] - ppt download](https://slideplayer.com/16216527/95/images/slide_1.jpg)

![log(10-14) = log[H+]+ log[OH-] -14 = -pH log(10-14) = log[H+]+ log[OH-] -14 = -pH](https://s3.studylib.net/store/data/008348689_1-ecdbf98005ad825214c83510bb4d3705.png)
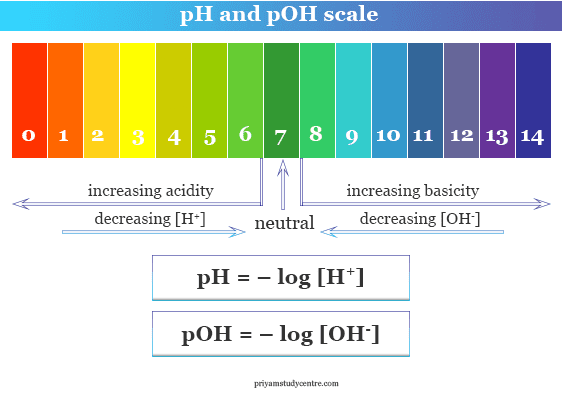
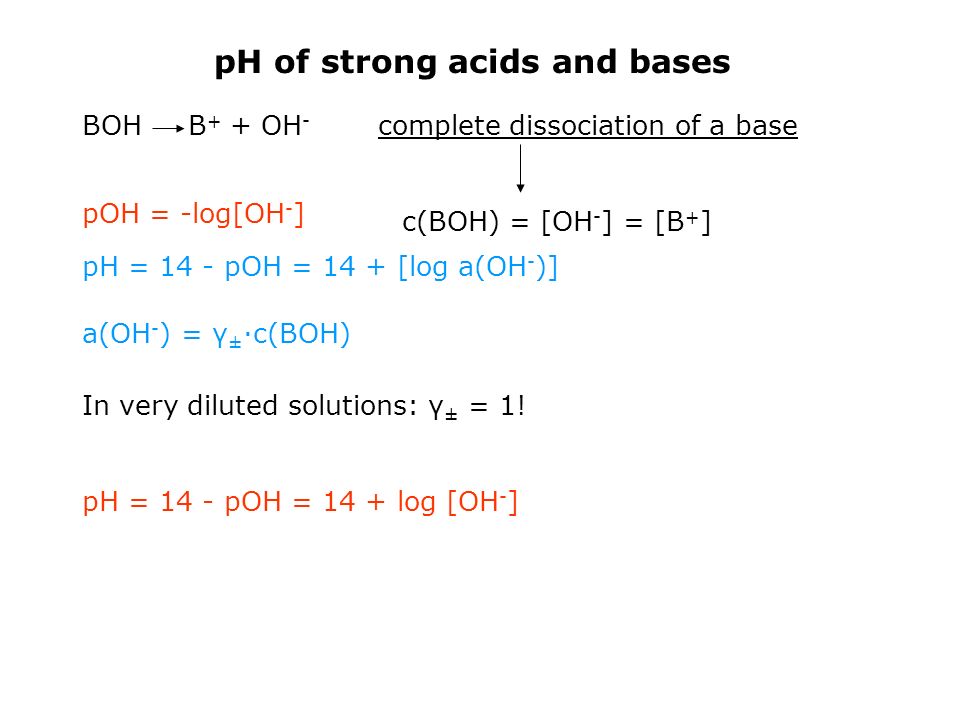
![Solved What is the pH of the following [H+]7.562E-7? pH = | Chegg.com Solved What is the pH of the following [H+]7.562E-7? pH = | Chegg.com](https://media.cheggcdn.com/media/140/140a4b7d-c635-44b7-b8be-db4be9aaac74/phpEEGmtT)
![Solved Equations: Kw = [H+][OH-] Ky = 1.0 x 10-14 pH = | Chegg.com Solved Equations: Kw = [H+][OH-] Ky = 1.0 x 10-14 pH = | Chegg.com](https://media.cheggcdn.com/study/9d2/9d2152c1-8645-4844-b8f4-97b10b603a5b/image.png)
![Finding the pH, pOH, [H+], [OH-] - ACIDS AND BASES: IT'S ACTUALLY Quite "BASIC" Finding the pH, pOH, [H+], [OH-] - ACIDS AND BASES: IT'S ACTUALLY Quite "BASIC"](http://itsactuallyquitebasic.weebly.com/uploads/2/7/8/0/27808159/4950515.png?357)
![Solving For pH, pOH, [H+], [OH-] - Acids & Bases Solving For pH, pOH, [H+], [OH-] - Acids & Bases](http://youarebasic.weebly.com/uploads/5/0/1/4/50143245/5802360_orig.png)
