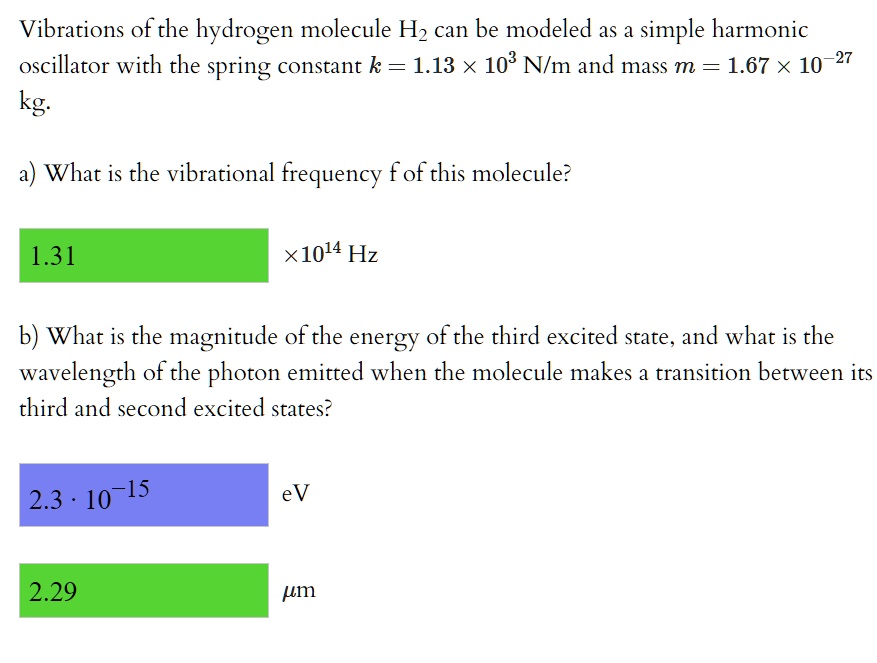
SOLVED: Vibrations of the hydrogen molecule H2 can be modeled as a simple harmonic oscillator with the spring constant k = 1.13 x 103 Nlm and mass m = 1.67 X 10-27
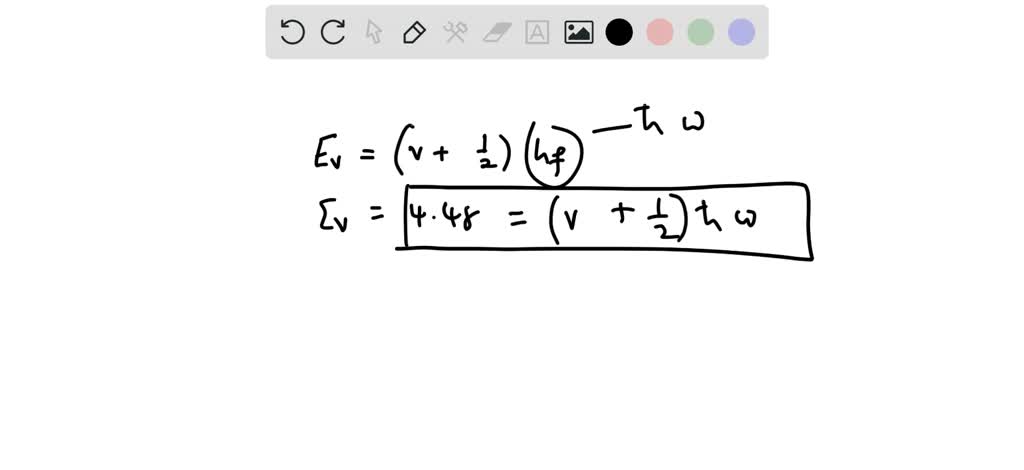
SOLVED: (15 points) The hydrogen molecule has a dissociation energy (De) 0f457.8 kJlmol and a vibrational frequency of 1.295 1014 s-1 . Assume that the ground electronic state is singly degenerate Hydrogen's
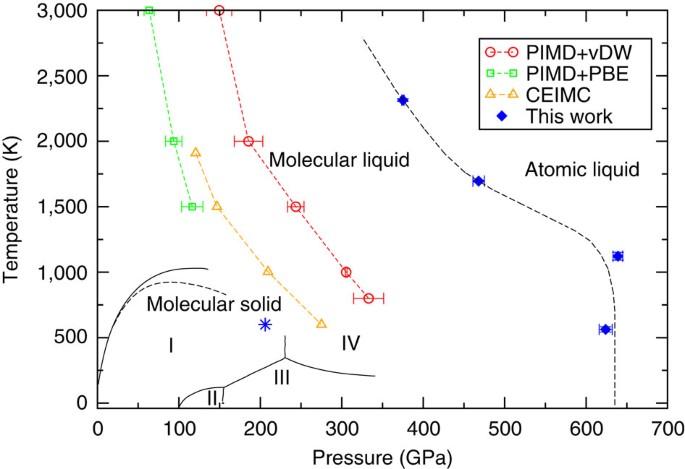
Unexpectedly high pressure for molecular dissociation in liquid hydrogen by electronic simulation | Nature Communications


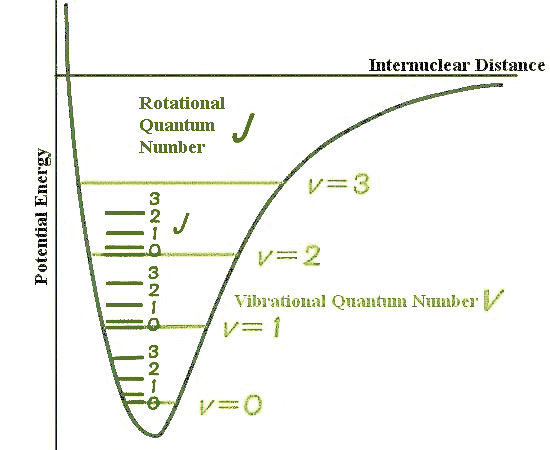


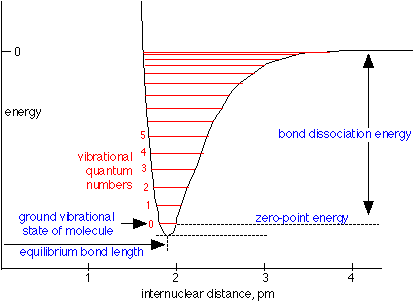

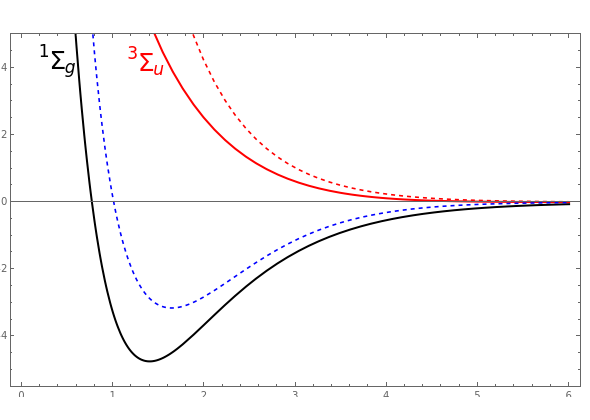
![PDF] Dissociation energies of molecular hydrogen and the hydrogen molecular ion. | Semantic Scholar PDF] Dissociation energies of molecular hydrogen and the hydrogen molecular ion. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/12ffde1ca3347126c0eb5ffc80a1cdd4785f9bf3/1-Figure1-1.png)
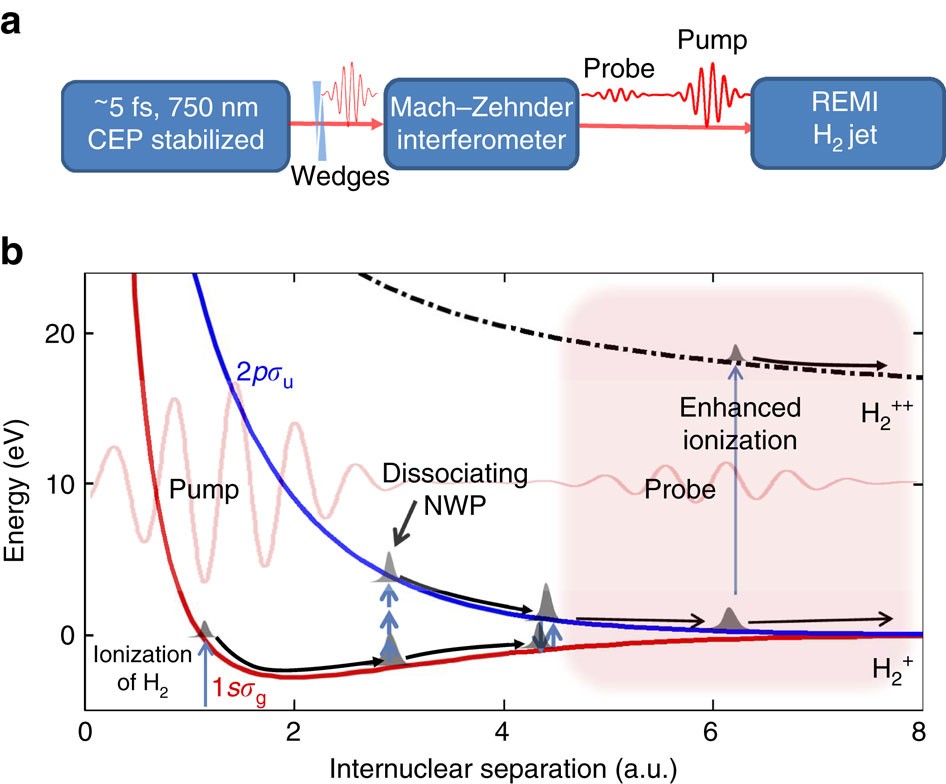
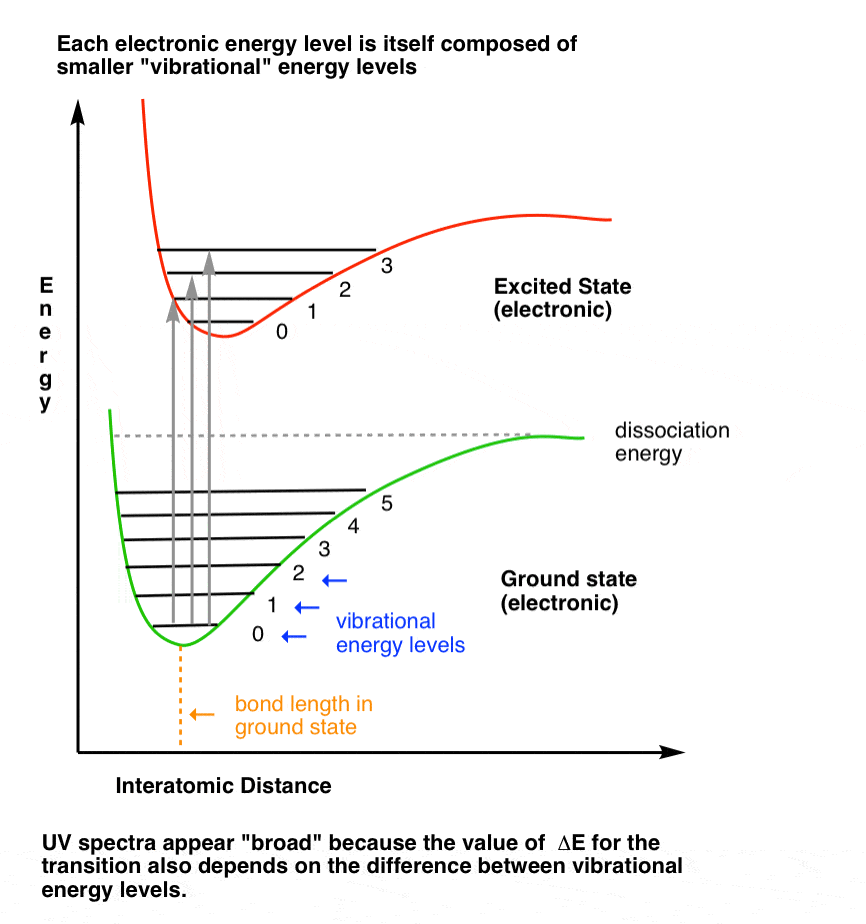

![PDF] Dissociation energies of molecular hydrogen and the hydrogen molecular ion. | Semantic Scholar PDF] Dissociation energies of molecular hydrogen and the hydrogen molecular ion. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/12ffde1ca3347126c0eb5ffc80a1cdd4785f9bf3/3-Figure3-1.png)
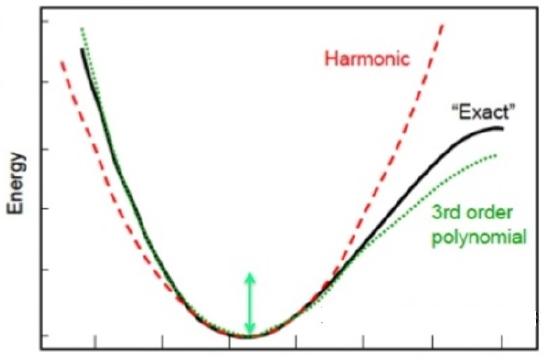

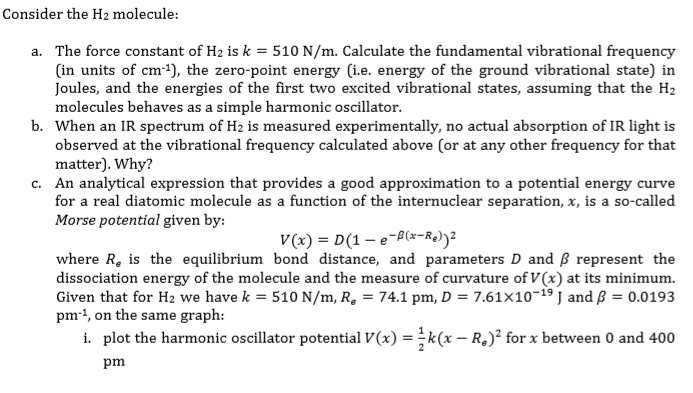

![PDF] Dissociation energies of molecular hydrogen and the hydrogen molecular ion. | Semantic Scholar PDF] Dissociation energies of molecular hydrogen and the hydrogen molecular ion. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/12ffde1ca3347126c0eb5ffc80a1cdd4785f9bf3/4-TableIII-1.png)