
Recording Adverse Events Following Joint Arthroplasty: Financial Implications and Validation of an Adverse Event Assessment Form - ScienceDirect

Identifying adverse drug event information in clinical notes with distributional semantic representations of context - ScienceDirect

National and institutional trends in adverse events over time: a systematic review and meta-analysis of longitudinal retrospective patient record review studies
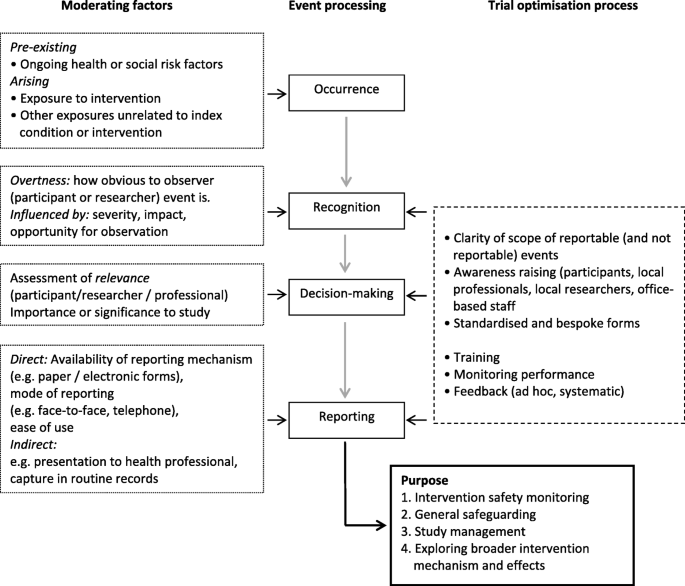
Monitoring adverse social and medical events in public health trials: assessing predictors and interpretation against a proposed model of adverse event reporting | Trials | Full Text

Rates of laboratory adverse events by course in paediatric leukaemia ascertained with automated electronic health record extraction: a retrospective cohort study from the Children's Oncology Group - The Lancet Haematology
![PDF] Assessment of Adverse Events in Protocols, Clinical Study Reports, and Published Papers of Trials of Orlistat: A Document Analysis | Semantic Scholar PDF] Assessment of Adverse Events in Protocols, Clinical Study Reports, and Published Papers of Trials of Orlistat: A Document Analysis | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6eb4cfb36ac5c33c4a0041b9493b10c576b73e7f/9-Table3-1.png)
PDF] Assessment of Adverse Events in Protocols, Clinical Study Reports, and Published Papers of Trials of Orlistat: A Document Analysis | Semantic Scholar









