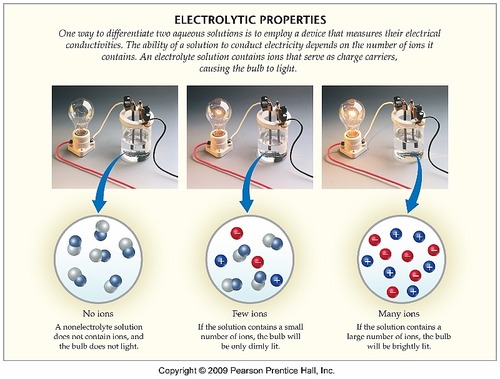
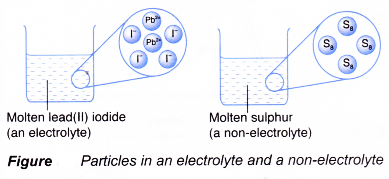
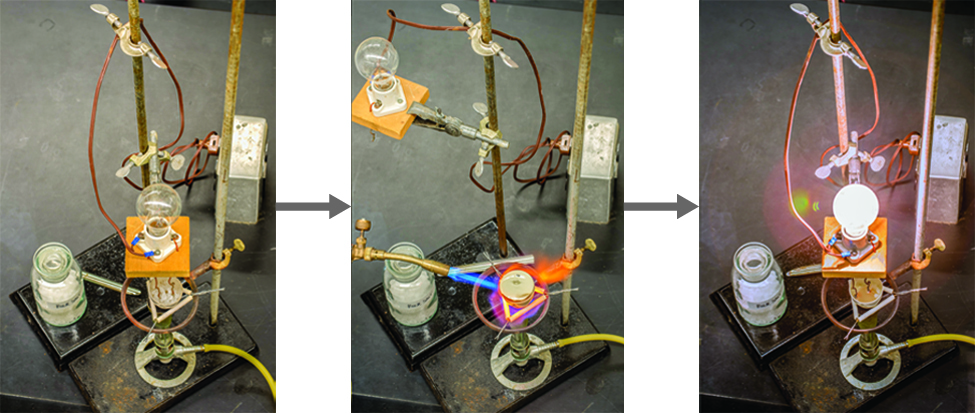
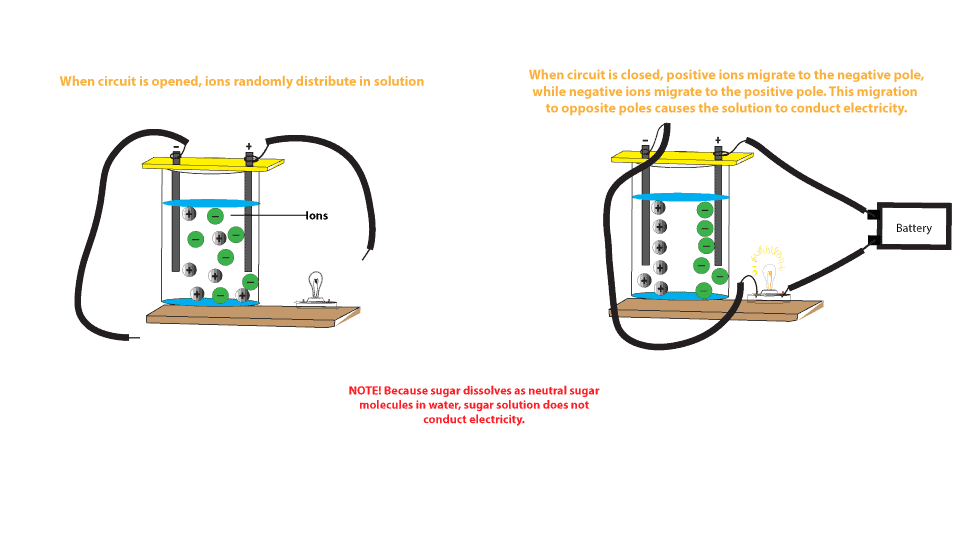
On Target? Do this on your Warm Up worksheet! Why does sugar dissolved in water not conduct electricity, while salt dissolved in water does conduct electricity? - ppt download

Explain the following : (a) Sodium chloride is an ionic compound which does not conduct electricity in solid state whereas it does conduct electricity in molten state as well as in aqueous

NaCl is not a conductor of electricity in solid state whereas it does conduct electricity in aqueous solution as well as in molten state.

√ A solid salt crystal is in insulator. Explain how salts can be made to conduct electricity - YouTube

Question Video: Identifying the Substance That Does Not Conduct Electricity in a Set of Giant Structures | Nagwa
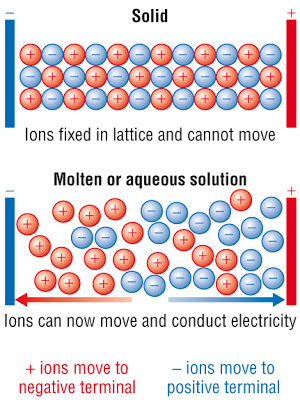
Why don't ionic compounds have electrical conductivity as a solid but they do as a liquid? | Socratic

Give reasons why :(i) Sodium Chloride will conduct electricity only in fused or aqueous solution state.(ii) In the electroplating of an article with silver, the electrolyte sodium argento - cyanide solution is
Why does solid sodium chloride act as a non-electrolyte while an aqueous NaCl solution acts as a strong electrolyte? - Quora